CRISPR Unpacked: Promises, Successes and Roadblocks
When the gene-editing potential of CRISPR/Cas9 was elucidated by Emmanuelle Charpentier and Jennifer Doudna in 2012 (1), it marked a turning point for precision medicine: for the first time, the prospect of correcting DNA at its source offered the potential for lasting cures to genetic diseases. CRISPR gene editing uses a guide RNA to direct Cas9 enzyme to a specific DNA sequence, where it makes a precise cut to delete or replace genetic material (FIG. 1). CRISPR-based DNA editing was first attempted for therapeutic use in humans between 2014 and 2018. Since then, a surge of biotech startups has emerged, aiming to harness this gene-editing tool. In 2023, the FDA approved Casgevy, the first clinically approved CRISPR-based gene-editing therapy for sickle cell disease. Casgevy utilizes CRISPR/Cas9 technology to edit a patient’s own hematopoietic (blood-forming) stem cells. The process involves collecting the patient’s stem cells, editing them ex vivo to increase the production of fetal hemoglobin, and then reintroducing them into the patient. While the promise is immense, translating CRISPR into successful in vivo therapies has proven challenging. A major hurdle lies in the inherent risks of cutting DNA, CRISPR’s hallmark mechanism. Off-target edits or unintended double-strand breaks can lead to permanent genomic damage, with potentially fatal consequences such as chromosome rearrangements and cancer. Additionally, delivering the CRISPR machinery safely and effectively to target tissues in vivo remains a major obstacle. As a result, despite the initial excitement, only a handful of companies have managed to advance CRISPR-based treatments to clinical trials (2).
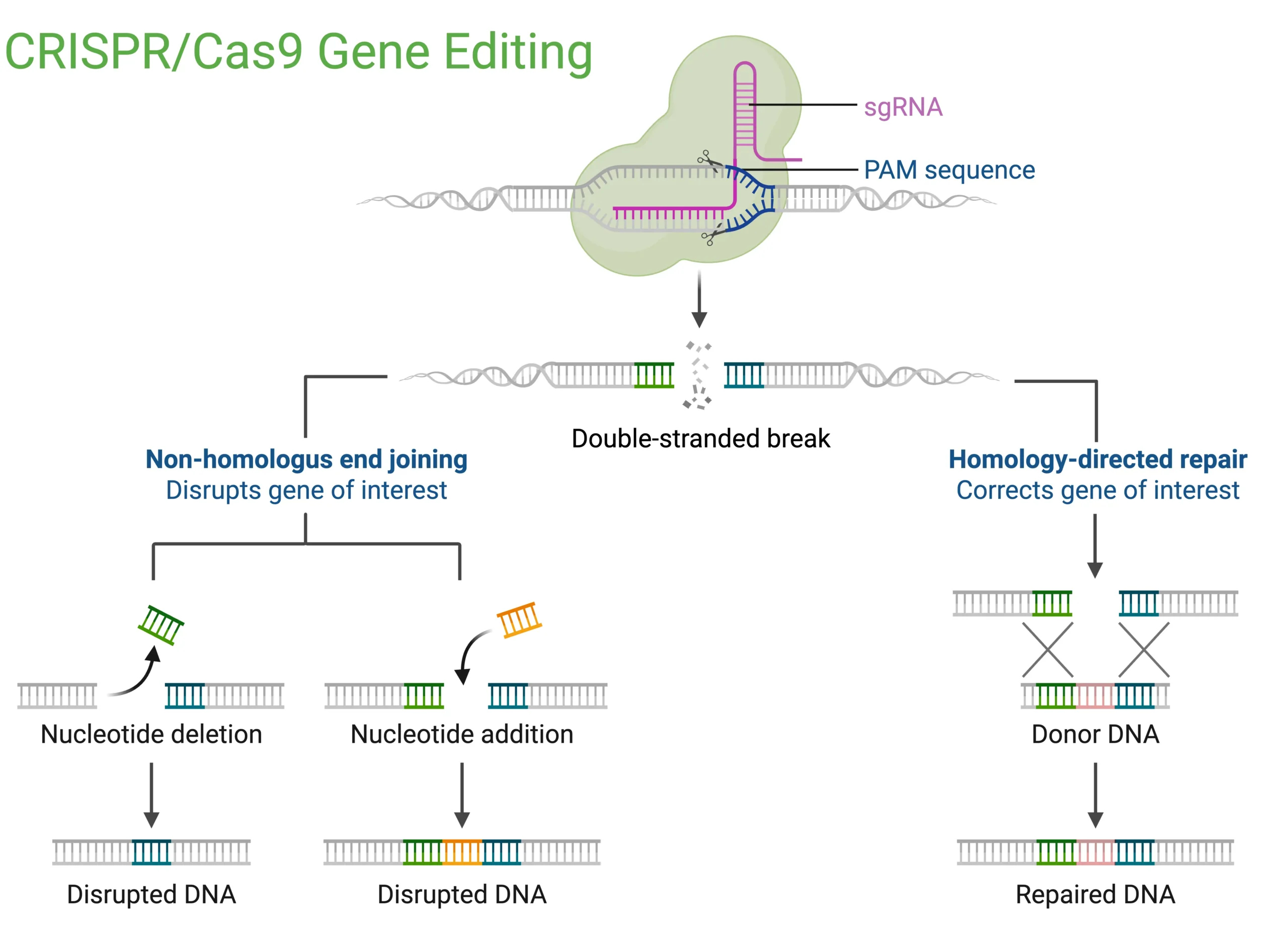
Figure 1: Schematic of CRISPR/Cas9 gene editing. A single-guide RNA (sgRNA) directs Cas9 to a PAM-adjacent genomic locus where it induces a double-strand break. Repair by error-prone non-homologous end joining introduces insertions or deletions that disrupt the gene, while homology-directed repair using a donor DNA template precisely corrects the sequence.
The Emergence of RNA Editing
Concerns over the safety of CRISPR-based DNA editing prompted researchers to explore RNA as a more transient and potentially safer substrate. This shift led to the development of CRISPR-based RNA editing systems, notably the REPAIR system (RNA Editing for Programmable A-to-I Replacement) introduced in 2016 by Feng Zhang’s lab at the Broad Institute (3). REPAIR utilizes catalytically inactive Cas13 (dCas13) fused to the deaminase domain of an ADAR (Adenosine Deaminases Acting on RNA) enzyme. dCas13 retains its ability to recognize and bind specific RNA sequences guided by a CRISPR RNA, but lacks nuclease activity due to point mutations in its catalytic domain. The fused ADAR enzyme converts adenosine (A) to inosine (I) in the targeted RNA transcripts, effectively altering protein output without modifying the underlying DNA. Inosine is a modified RNA base that is read as a guanosine (G) by the cellular machinery. Between 2018 and 2020, advancements like RESCUE and other Cas13-based systems enabled C-to-U RNA edits, increasing the breadth of codon changes that could be realized (4). However, these CRISPR-based RNA editing systems remained large and therefore challenging to deliver efficiently to specific cells in vivo, limiting their therapeutic application.
Harnessing Endogenous ADAR for Safer RNA Editing
ADARs are endogenous enzymes found in human cells that catalyze the conversion of adenosine (A) to inosine (I) within double-stranded RNA regions (5) (FIG. 2). Because the cellular machinery reads inosine as guanosine (G), editing of the coding regions of mRNA results in a codon change, altering the amino acid sequence of the protein. Additionally, A-to-I editing can affect splicing patterns and RNA stability. Antisense oligo (ASO)-based RNA editing takes advantage of this natural machinery. Instead of introducing foreign proteins, researchers use ASOs—short, single-stranded, synthetic RNA or DNA strands—that bind to the target mRNA, forming a short double-stranded structure that recruits endogenous ADAR to perform site-specific A-to-I editing. The method was first reported by Thorsten Stafforst in 2019 under the name RESTORE (recruiting endogenous ADAR to specific transcripts for oligonucleotide-mediated RNA editing), and shortly after by Wensheng Wei as LEAPER (leveraging endogenous ADAR for programmable editing of RNA) (6, 7). Because ADAR-catalyzed editing is limited to the ASO binding site, this method promises fewer off-target effects and overall higher specificity. The safety profile is desirable because no foreign proteins are introduced into the body and the transient nature of RNA eliminates the risk of permanent, unintended genome alterations. Moreover, ASOs benefit from well-established delivery methods, such as subcutaneous or intrathecal injection, and naturally accumulate in key tissues like the liver, muscle, and central nervous system where ADAR is endogenously active (8).
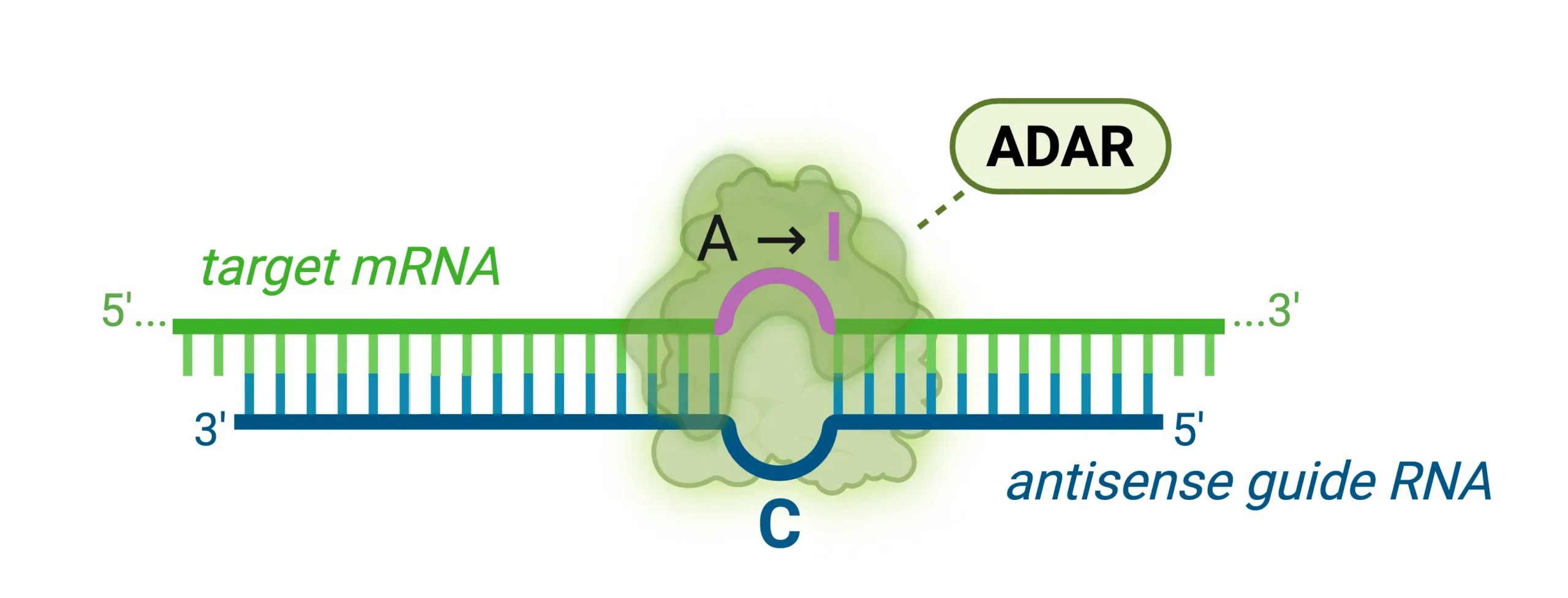
Figure 2: Schematic of ADAR-mediated site-specific RNA editing. An antisense guide RNA binds the target mRNA to form a short duplex that recruits endogenous ADAR, which deaminates adenosine to inosine; the translation machinery reads inosine as guanosine, potentially resulting in a codon change.
Commercialization and Optimization of ASO-Based Editing
Several companies are working on harnessing ASO-based RNA editing for therapeutic applications, including ProQR Therapeutics, Korro Bio, ADARx Pharmaceuticals and Wave Life Sciences. The companies are differentiated in their disease indications and targets and the design of ASOs. Optimizing ASOs for RNA editing involves several key strategies to enhance their precision, stability, and efficiency. These strategies target the chemical structure of the ASO backbone, ribose sugar and other features for ADAR recruitment. The ASO backbone is critical for ASO stability. For example, incorporating phosphorothioates or changing the stereochemistry can significantly influence pharmacological behavior. Additionally, sugar modifications at the 2’OH position help increase binding affinity, protect against nuclease degradation, and fine-tune RNA interactions, thereby enhancing ADAR recruitment. Another refinement is the use of a central mismatch opposite the target adenosine in the ASO-RNA duplex, which has been shown to boost editing efficiency by promoting ADAR activity at the desired site. Lastly, targeted delivery enhancements, like conjugating ASOs with ligands that enable selective uptake in tissues of interest, maximize therapeutic impact while minimizing systemic exposure (8).
Beyond A-to-I: The Future for RNA Editing
While the majority of RNA editing schemes employ A-to-I editing to correct point mutations in DNA that are passed on to mRNA, other RNA editing schemes are gradually emerging. One example is site-specific pseudouridinylation, a concept that was first described in a patent by Yi-Tao Yu in 1998, long before anybody envisioned therapeutic RNA editing. His method uses engineered small nucleolar RNAs (snoRNAs) to direct pseudouridylation of specific uridine residues (9). Even though pseudouridinylation does not change a codon, it modulates the behavior of the ribosome. Yu’s work has demonstrated that targeted pseudouridylation of premature stop codons causes the ribosome to skip over the stop signal. Thus, targeted pseudouridylation offers a promising approach to treat diseases caused by premature stop codons by converting specific uridines to pseudouridines in mRNA, promoting translational readthrough and restoring full-length protein production; examples of such diseases include Duchenne muscular dystrophy, cystic fibrosis, and Hurler syndrome.
RNA Modifications and the Subtle Art of Precision Gene Control
In addition to pseudouridine and inosine, other naturally occurring RNA modifications—such as N6-methyladenosine (m6A) and 5-methylcytosine (m5C)—are gaining attention for their regulatory roles. m6A, the most abundant internal mRNA modification in humans, influences RNA stability, splicing, translation, and localization. Its dynamic regulation by “writer,” “eraser,” and “reader” proteins offers an opportunity to fine-tune gene expression post-transcriptionally. Researchers are now engineering programmable RNA-targeting systems using tools like dCas13 fused to m6A regulators to modulate these marks with precision (10-15). This approach adds a powerful, reversible layer of gene control that avoids permanently altering genome sequences —ideal for therapeutic use where safety is paramount.
Conclusion: Toward a Gentler Genetic Medicine
As the field moves beyond DNA cuts toward reversible RNA edits, the future of gene therapy may not lie in rewriting our genetic code, but in tuning its expression. Therapeutic RNA editing, particularly approaches that leverage endogenous enzymes, offers a compelling alternative to CRISPR by sidestepping the risks of permanent DNA modification. As tools for targeting and modifying RNA become more refined, a new generation of treatments is emerging: safer, more precise, and deeply attuned to the dynamic nature of biology.
Explore other posts in our Mods in Motion series
Blog posts
-
A Beginner’s Guide to m6A and Other RNA Modifications
N6-methyladenosine (m6A): Minor Modification, Major Impact
- Podcasts
What Are Those RNA Modifications Doing for Gene Regulation?
The hidden language of RNA – how epigenetics is shaping medicine
About the Author
Gudrun Stengel
Gudrun Stengel is the CEO and scientific co-founder of AlidaBio. Gudrun specializes in developing genomics platforms, drawing on her experience in NGS development and background in biophysics and biochemistry. She has made significant contributions to the field, including sequencing chemistries for the HiSeqX and NovaSeq platforms at Illumina, and the AVITI platform at Element. Gudrun was a postdoctoral researcher at the Scripps Research Institute and the University of Colorado Boulder, studying the molecular mechanisms of DNA replication and transcription. Altogether Gudrun published more than 20 peer-reviewed articles. She received a Masters of Biochemistry and Ph.D. in Biophysical Chemistry from the Max Planck Institute in Germany.
References
(1) Jinek, M., Chylinski, K., Fonfara, I., Hauer, M., Doudna, J. A., & Charpentier, E. (2012). A Programmable Dual-RNA–Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science, 337(6096), 816–821.
(2) Li, T., Yang, Y., Qi, H., et al. (2023). CRISPR/Cas9 therapeutics: progress and prospects. Signal Transduction and Targeted Therapy, 8, 36.
(3) Cox, D. B. T., Gootenberg, J. S., Abudayyeh, O. O., Franklin, B., Kellner, M. J., Joung, J., & Zhang, F. (2017). RNA editing with CRISPR-Cas13. Science, 358(6366), 1019–1027.
(4) Abudayyeh, O. O., Gootenberg, J. S., Franklin, B., Koob, J., Kellner, M. J., Ladha, A., … & Zhang, F. (2019). A cytosine deaminase for programmable single-base RNA editing. Science, 365(6451), 382-386.
(5) Nishikura, K. (2016). A-to-I editing of coding and non-coding RNAs by ADARs. Nature Reviews Molecular Cell Biology, 17(2), 83–96.
(6) Merkle, T., Merz, S., Reautschnig, P., Blaha, A., Li, Q., Vogel, P., Wettengel, J., Li, J. B., & Stafforst, T. (2019). Precise RNA editing by recruiting endogenous ADARs with antisense oligonucleotides. Nature Biotechnology, 37(2), 133–138.
(7) Qu, L., Yi, Z., Zhu, S., Wang, C., Cao, Z., Zhou, Z., Yuan, P., Yu, Y., Tian, F., Liu, Z., & Bao, G… (2019). Programmable RNA editing by recruiting endogenous ADAR using engineered RNAs. Nature Biotechnology, 37(9), 1059–1069.
(8) Booth, B. J., Nourreddine, S., Katrekar, D., Savva, Y., Bose, D., Long, T. J., Huss, D. J., & Mali, P. (2023). RNA editing: Expanding the potential of RNA therapeutics. Molecular Therapy, 31(6), 1533–1549.
(9) Zhao, X., Li, Z.-H., Terns, R. M., Terns, M. P., & Yu, Y.-T. (2002). An H/ACA guide RNA directs U2 pseudouridylation at two different sites in the branch point recognition region in Xenopus oocytes. RNA, 8(12), 1515–1525.
(10) Liu, X. M., Zhou, J., Mao, Y., Ji, Q. & Qian, S. B. Programmable RNA N(6)-methyladenosine editing by CRISPR-Cas9 conjugates. Nat. Chem. Biol. 15, 865–871 (2019).
(11) Wilson, C., Chen, P. J., Miao, Z. & Liu, D. R. Programmable m(6)A modification of cellular RNAs with a Cas13-directed methyltransferase. Nat. Biotechnol. 38, 1431–1440 (2020).
(12) Zhao, J., Li, B., Ma, J., Jin, W. & Ma, X. Photoactivatable RNA N(6) -methyladenosine editing with CRISPR-Cas13. Small 16, e1907301 (2020).
(13) Li, J. et al. Targeted mRNA demethylation using an engineered dCas13b-ALKBH5 fusion protein. Nucleic Acids Res. 48, 5684–5694 (2020).
(14) Xia, Z. et al. Epitranscriptomic editing of the RNA N6-methyladenosine modification by dCasRx conjugated methyltransferase and demethylase. Nucleic Acids Res. 49, 7361–7374 (2021).
(15) Rauch, S., He, C. & Dickinson, B. C. Targeted m(6)A reader proteins to study epitranscriptomic regulation of single RNAs. J. Am. Chem. Soc. 140, 11974–11981 (2018).