

One of the most transformative post-transcriptional modifications is adenosine-to-inosine (A-to-I) RNA editing – a chemical change that alters base-pairing preference and modifies how RNA is read, processed, and translated. This form of editing, catalyzed by ADAR enzymes (adenosine deaminase acting on RNA), introduces inosine into RNA, allowing a single nucleotide to carry functional consequences across diverse biological processes. Understanding the scope and specificity of A-to-I editing provides insights into fundamental cellular mechanisms and opens new windows into disease pathology. In this post, we chart the molecular logic of A‑to‑I editing – from the enzymatic chemistry of ADARs to its prominent role in RNA regulation.
Inosine: A Subtle Substitution with Broad Consequences
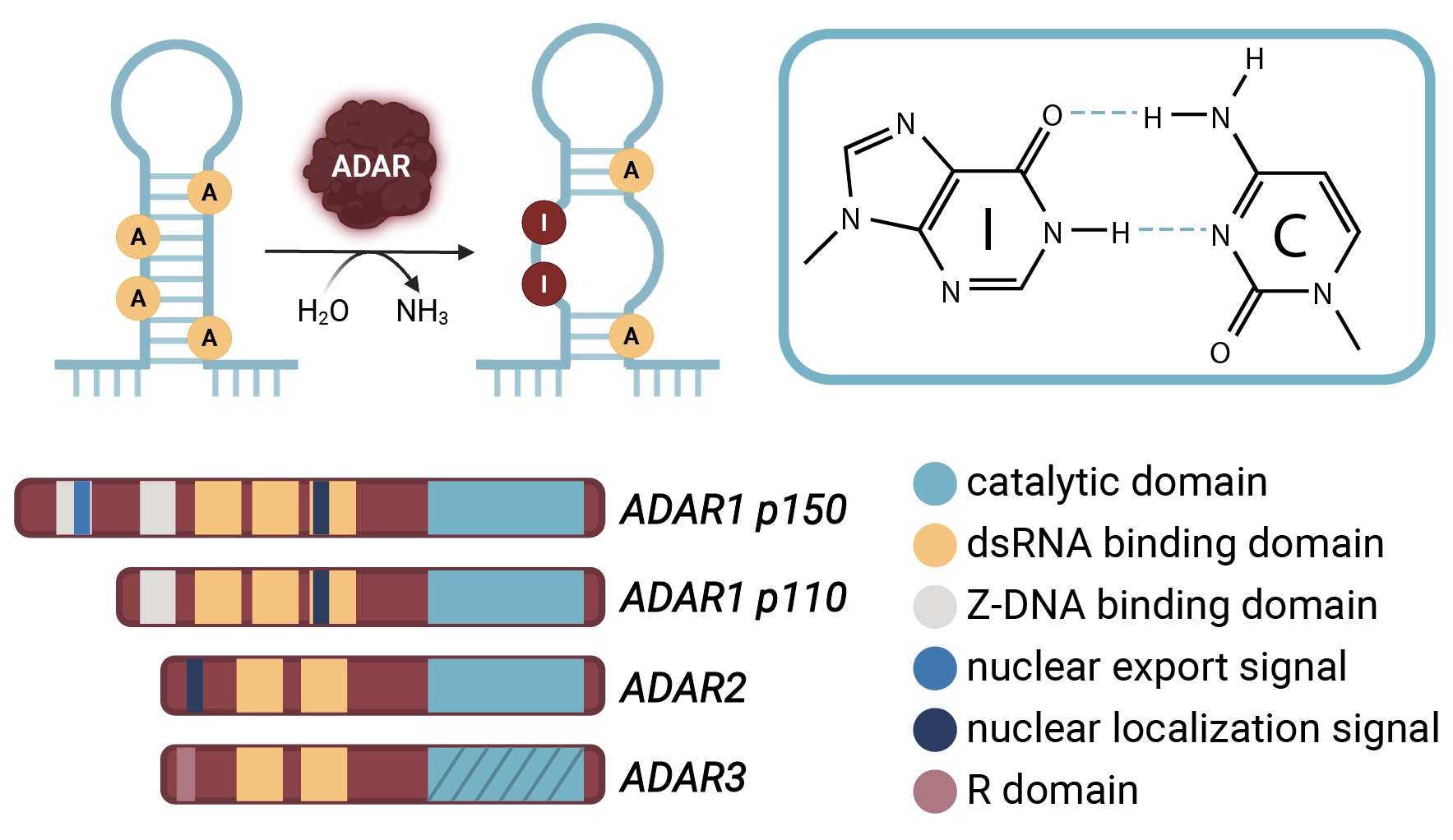
Inosine arises in RNA by the deamination of adenosine wherein an amine group is replaced by a carbonyl. Though this change appears minor, it fundamentally alters the base-pairing properties of the nucleotide. Inosine behaves like guanosine during base pairing, preferentially forming hydrogen bonds with cytidine. Consequently, A-to-I editing introduces an “effective” A-to-G substitution in the RNA sequence, without altering the genomic sequence.
The substitutions have significant implications. In coding sequences, inosine is interpreted by both the ribosome and reverse‑transcription enzymes as guanosine, so a single A‑to‑I event can recode an amino acid and appear as an A→G mismatch in RNA‑seq data. In non‑coding regions, inosine reshapes RNA folding landscapes: clusters of edits (so‑called “hyper‑editing”) within paired Alu repeats are weakened by I‑C versus A‑U hydrogen bonding, locally melting double helices. This modulates access for RNA‑binding proteins, triggering nuclear retention or promoting rapid cytoplasmic turnover and giving cells a tunable handle on transcript stability.1 Inosine‑rich dsRNAs also dampen the innate immune response by disrupting the contiguous base‑pairing, illustrating how a solitary chemical swap reverberates from atomic‑scale bonding to organism‑level immune tolerance.2 Latent G-quadruplexes, higher ordered structures in guanosine-rich regions, can be reactivated by A-to-I edits that fill in incomplete G-quadruplexes with inosine having implications to mRNA localization, splicing regulation and compaction into stress granules.3,4 Together, these multifaceted effects explain the diverse phenotypic outcomes of A‑to‑I editing across cell types and biological contexts.
The Editors: ADAR Enzymes and Their Substrates
The conversion of adenosine to inosine in RNA is catalyzed by a conserved family of enzymes known as adenosine deaminases acting on RNA (ADARs). In humans, three ADAR proteins have been identified: ADAR1, ADAR2, and ADAR3. Both ADAR1 and ADAR2 possess catalytic activity, whereas ADAR3 lacks deaminase function and competes with ADAR1 and ADAR2 for RNA binding sites, thereby modulating substrate access and mRNA stability.5
ADARs act preferentially on double‑stranded RNA (dsRNA) substrates, which may arise from inverted repeat elements, structured untranslated regions (UTRs), or pre‑mRNA stem‑loop structures. Two major ADAR1 isoforms exist: p110, constitutively expressed and found predominantly in the nucleus, and p150, an interferon‑inducible form that shuttles between nucleus and cytoplasm. ADAR1‑p150 dominates the editing of repetitive Alu duplexes and is essential for the innate immune response to viral infections.6
ADAR2, in contrast, exhibits exquisite site selectivity for a limited cohort of transcripts enriched in the nervous system, guided by local stem‑loop cues and auxiliary RNA‑binding proteins. An example is in the GluA2 (GRIA2) subunit of the AMPA ionotropic glutamate receptor, a fast-acting neuronal ion channel, where ADAR2 editing converts a glutamine (Q) codon to arginine (R). This recoding mutation lowers Ca²⁺ permeability and protects neurons from cell death due to overexcitation.7 Mice lacking ADAR2 or harboring uneditable GluA2 alleles exhibit lethal seizures, underscoring the physiological relevance of this single RNA modification.1
Summary of ADAR Proteins
Biological Functions of Inosine in RNA Regulation
A-to-I editing modulates RNA behavior across several dimensions – coding potential, splicing, structural dynamics, localization, and immune tolerance – each of which can profoundly influence cellular phenotype.
As described above, one of the most striking consequences is protein recoding. While the majority of editing occurs in non-coding regions, select edits in coding regions can change amino acid identity, alter protein stability or localization, and generate functional isoforms. For instance, in addition to GRIA2, other edited transcripts include serotonin receptor 2C (HTR2C), potassium channel Kv1.1, and various G-protein–coupled receptors, all of which demonstrate altered signaling or electrophysiological properties depending on their editing status.8,9
Editing can also affect RNA splicing. Inosines are interpreted as guanosines by the splicing machinery, and editing near splice sites can lead to exon skipping or inclusion, modulating transcript isoforms. Intriguingly, ADAR2 edits its own pre‑mRNA creating a novel 3’ splice site, triggering alternative splicing and downregulation of the enzyme’s own expression – a feedback mechanism that exemplifies the tight regulation of this system.10
Beyond splicing, inosine can influence RNA stability and nuclear export. Perfectly base-paired dsRNAs are often retained in the nucleus or targeted for degradation; editing introduces mismatches that can destabilize the helix and facilitate export. In hyperedited RNAs, however, excessive inosine content can lead to recognition by degradation pathways, such as those involving Tudor-SN or other inosine-binding nucleases.6
In microRNA, A-to-I editing affects both sides of the regulatory axis: editing of pri- or pre-miRNAs (precursors to mature miRNAs) can alter Dicer processing or RISC loading, while editing of miRNA target sites in mRNAs can prevent silencing or redirect regulation to alternative targets.11
Inosine’s Lasting Imprint
A‑to‑I editing is now recognized as a molecular “pivot,” subtly swapping an A for a functionally disguised G to fine‑tune RNA structure and coding potential. The next decade will likely see clinical sequencing pipelines incorporate editing signatures as targeted ADAR editing technologies move toward the clinic. Continued work to map the epitranscriptome and understand editing crosstalk with other RNA modifications, and the interplay across the entire “multiome”, will be critical. Inosine editing thus stands not only as a fascinating biological mechanism but also as a burgeoning toolkit for diagnostics, therapeutics, and evolutionary insight.
Explore additional posts in our Mods in Motion series
Blog Posts
- No Cuts, Just Edits: Therapeutic A-to-I Editing as a Safer Alternative to CRISPR’s Scalpel
- A Beginner’s Guide to m6A and Other RNA Modifications
Podcasts
About the Author
Zachary Miles
Zachary Miles is the Sr. Director of Protein Engineering at AlidaBio. He earned a PhD in biochemistry at the University of Arizona studying biosynthetic pathways and enzymatic mechanisms of RNA modification. A postdoctoral position at The University of California – San Diego exposed him to the extensive biotechnology ecosystem where he then utilized this expertise in protein structure/function to help design enzymes and proteins at multiple companies in the San Diego area.
References
- Bass, B. L. (2002). RNA Editing by Adenosine Deaminases That Act on RNA. Annual Review of Biochemistry, 71(1), 817–846. https://doi.org/10.1146/annurev.biochem.71.110601.135501
- Mendoza, H. G., & Beal, P. A. (2024). Structural and functional effects of inosine modification in mRNA. RNA (New York, N.Y.), 30(5), 512–520. https://doi.org/10.1261/rna.079977.124
- Hagen, T., Laski, A., Brümmer, A., Pruška, A., Schlösser, V., Cléry, A., Allain, F. H.-T., Zenobi, R., Bergmann, S., & Hall, J. (2021). Inosine Substitutions in RNA Activate Latent G-Quadruplexes. Journal of the American Chemical Society, 143(37), 15120–15130. https://doi.org/10.1021/jacs.1c05214
- Sahayasheela, V. J., & Sugiyama, H. (2024). RNA G-quadruplex in functional regulation of noncoding RNA: Challenges and emerging opportunities. Cell Chemical Biology, 31(1), 53–70. https://doi.org/10.1016/j.chembiol.2023.08.010
- Zhang, Y., Li, L., Mendoza, J. J., Wang, D., Yan, Q., Shi, L., Gong, Z., Zeng, Z., Chen, P., & Xiong, W. (2024). Advances in A-to-I RNA editing in cancer. Molecular Cancer, 23(1), 280. https://doi.org/10.1186/s12943-024-02194-6
- Nishikura, K. (2016). A-to-I editing of coding and non-coding RNAs by ADARs. Nature Reviews Molecular Cell Biology, 17(2), 83–96. https://doi.org/10.1038/nrm.2015.4
- Higuchi, M., Maas, S., Single, F. N., Hartner, J., Rozov, A., Burnashev, N., Feldmeyer, D., Sprengel, R., & Seeburg, P. H. (2000). Point mutation in an AMPA receptor gene rescues lethality in mice deficient in the RNA-editing enzyme ADAR2. Nature, 406(6791), 78–81. https://doi.org/10.1038/35017558
- Burns, C. M., Chu, H., Rueter, S. M., Hutchinson, L. K., Canton, H., Sanders‑Bush, E., & Emeson, R. B. (1997). Regulation of serotonin‑2C receptor G‑protein coupling by RNA editing. Nature, 387(6630), 303–308. https://doi.org/10.1038/387303a0
- Bhalla, T., Rosenthal, J. J., Holmgren, M., & Reenan, R. (2004). Control of human potassium channel inactivation by editing of a small mRNA hairpin. Nature structural & molecular biology, 11(10), 950–956. https://doi.org/10.1038/nsmb825
- Rueter, S. M., Dawson, T. R., & Emeson, R. B. (1999). Regulation of alternative splicing by RNA editing. Nature, 399(6731), 75–80. https://doi.org/10.1038/19992
- Kawahara, Y., Zinshteyn, B., Chendrimada, T. P., Shiekhattar, R., & Nishikura, K. (2007). RNA editing of the microRNA-151 precursor blocks cleavage by the Dicer-TRBP complex. EMBO reports, 8(8), 763–769. https://doi.org/10.1038/sj.embor.7401011
